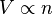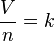Call 1800-123-2003

"Smartindia is the best online tool for school students." Ms Deepa Chandran, Principal Anchal
"Smartindia is one of the best supplementary education tool for school students." Ms Pooja, Principal
A small short note of Avogadro and his equations? |
Avogadro's law is an experimental gas law relating volume of a gas to the amount of substance of gas present. A modern statement of Avogadro's law is:
which can be written as: or where:
|