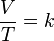Call 1800-123-2003

"Smartindia is the best online tool for school students." Ms Deepa Chandran, Principal Anchal
"Smartindia is one of the best supplementary education tool for school students." Ms Pooja, Principal
What is Charl's law |
| Charles's law states that: The volume of a given mass of an ideal gas is directly proportional to its temperature on the absolute temperature scale (in Kelvin) if pressure and the amount of gas remain constant; that is, the volume of the gas increases or decreases by the same factor as its temperature. This directly proportional relationship can be written as 
or
|