
Call 1800-123-2003
describe the formation of hydrogen, chlorine, nitrogen and oxygen with diagram? |
| Covalent Bonding: When non-metals react with non-metals they share electrons and form a covalent bond. Covalent means sharing. A covalent bond forms because of the electrostatic attraction between the nuclei of the atoms (that are positive) and the shared electrons (that are negative). There are no ions present (no + or - charges) because the electrons are shared, not transferred from one atom to another. One pair of shared electrons form a single covalent bond. Two pairs of shared electrons form a double covalent bond. Three pairs of shared electrons form a triple covalent bond. Formation of Hydrogen molecule: (1) A hydrogen atom has 1 electron in its outer shell. Hydrogen can only form 1 bond. 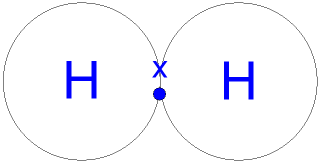 Formation of Chlorine molecule: (2,8,7) Chlorine is a non-metal. A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the periodic table. Two chlorine atoms will each share one electron to get a full outer shell and form a stable Cl2 molecule. 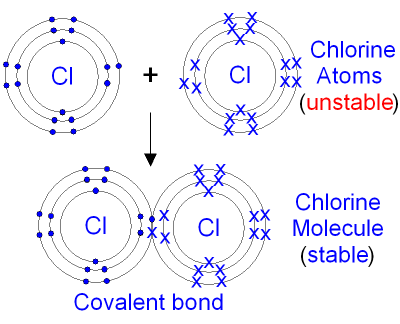 Formation of Oxygen molecule: (2,6) Oxygen is a non-metal. An oxygen atom has 6 electrons in its outer shell. Oxygen is in group 6 of the periodic table. Two oxygen atoms will each share two electrons to form two covalent bonds and make an oxygen molecule (O2). 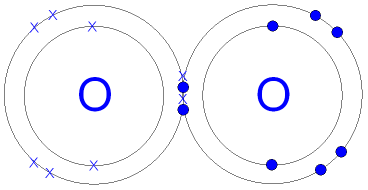 (2,6) (2,6) 2 pairs (4 electrons) shared between the atoms. Each electron pair is one bond. Oxygen has two bonds between its atoms. This is called a double bond. Formation of Nitrogen molecule: (2,5) Nitrogen is a non-metal. A nitrogen atom has 5 electrons in its outer shell. Nitrogen is in group 5 of the periodic table. Two nitrogen atoms will each share three electrons to form three covalent bonds and make a nitrogen molecule (N2). 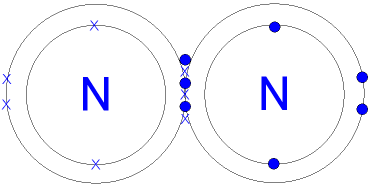 The structural formula of an nitrogen molecule is written |