
Call 1800-123-2003
what do you by confirmational isomers of ethane |
There are an infinite number of conformations about any sigma bond in ethane .Two particular conformers are namely staggered and eclipsed conformation .In the eclipsed conformation ,the C-H bonds on the front and back carbons are aligned with each other with dihedral angles of zero degrees .In the staggered conformation ,the C-H bonds on the rear carbon lie between those on the front carbon with dihedral angles of 60 degrees. 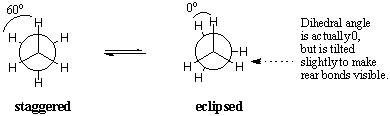 Energetically not all conformations are equally favoured .The eclipse conformation of ethane is less stable than the staggered conformation by 3 K cal/mol.The stagerred conformation is the most stable of all possible conformations of ethane .Since the angles between the C-H bonds on the front and rear carbon are maximised at 60 degrees .In the eclipsed form ,the electron densities on the C-H bonds are brought into a dihedral angle of zero degrees there with clouds experience repulsions which raises the energy of the molecule .The eclipse conformation of ethane has three such C-H eclipsing interactions .  |