
Call 1800-123-2003
explain the following a)pbcl4 is less stable than SnCl4 b)boric acid is monobasic non protic acid c)SnCL4 is good reducing agent d)BCl3 is better lewis acid than BF3 e)co2 is gas while silica is solid |
| a) The electronic configuration of group 14 elements is ns2 np2
.So the most common oxidation state exhibited by them should be +4 .+2
oxidation state is more and more common. On moving down the group the
higher oxidation state becomes less stable .This is because of the inert
pair effect .Thus Ge ,Sn,and Pb show both the +2 and +4 oxidation
states ,the stability of the lower oxidation state decreases on moving
down the group . C Si Ge Sn Pb > (stability of +2 state increases and +4 state decreases) Thus PbCl4 is less stable than SnCl4 . b) Boric acid is not a pro-tic acid .It is a weak mono basic acid ,behaving as a lewis acid. It behaves as an acid by accepting a pair of electrons from OH- ion . c) Substances which reduces the other substance and oxidises itself that is losses electrons and increases its on are reducing agents .A good reducing agent will easily oxidises itself and easily losses electrons . In the reaction , Cl2 + SnCl2 _________ > SnCl4 ,SnCl2 oxidised to SnCl4 and Cl2 reduced to Cl4 . So SnCl2 is a good reducing agent . d)Flo urine atoms have filled 2p orbitals that can overlap with the empty 2p orbital on the boron atom to give some double bond character to the B-F bonds .As a result of the contribution by resonance structures having double bond character ,the boron atom in BF3 is not as electron deficient as it is in BCl3 .So BCl3 is better lewis acid than BF3 . e)Carbondioxide is a gas but silicon dioxide is a hard melting solid ..This is because of the structural difference between carbon dioxide and dioxides of the rest of the group . CO2 is a gas because it consists of simple molecules .It can form simple molecules with oxygen because it can form double bonds with the oxygen . O=C=O Other elements in group 4 do not form double bonds with oxygen .So that forces completely different structures on them . When carbon forms bonds with oxygen ,it first promotes one of the electrons in the 2s level into the empty 2p level .This produces 4 unpaired electrons . 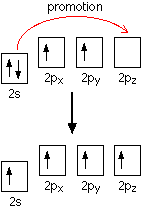 It now reshuffles those electrons slightly by hybridizing the 2s electron and one of the 2p electrons to make two sp1 hybrid orbitals of equal energy .The other 2p electrons are left alone . 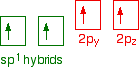 It can be seen like this in the atom ,  The two green lobes are two different hybrid orbitals arranged as far apart from each other as possible .The electronic structure of oxygen is 1s2 2s22px22py12pz1. Here sp2 hybrids are formed with the s orbital and two of the p orbitals being rearranged to give 3 orbitals of equal energy - leaving a temporarily unaffected p orbital . 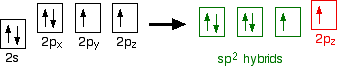 Here two of the sp2 hybrid orbitals contain lo0ne pairs of electrons .The diagrammatic representation for this can be shown like this , 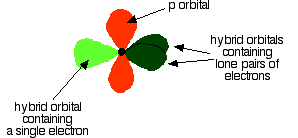 When line up the two oxygens and the carbon prior to bonding them it can be seen like this , 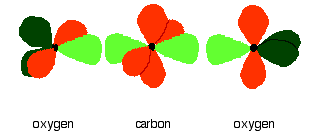 When bring them together ,the pale green hybrid orbitals overlap end to end form simple covalent bonds .These are properly called sigma bonds .This brings the various p orbitals close enough together that they overlap sideways like this , 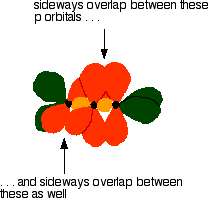 Side ways overlap between the two sets of two orbitals produces two pi bonds.This pi bonds are twisted at 90 0 each other in the final molecule like this ,  In the case of silicon dioxide ,silicon does not double bond with oxygen .Silicon atoms are bigger than carbon that is silicon-oxygen bonds will be longer than carbon-oxygen bonds . With the longer silicon-oxygen bonds ,the p orbitals on the silicon and the oxygen aren't quite close by allowing side ways overlap to give a stable pi bond . Silicon bonds with oxygen are single bonds .This can be shown like this , 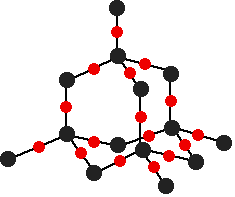 This is based on diamond structure with each of the silicon atoms being bridged to its other four neighbours via an oxygen atom . Silicon dioxide has a giant covalent structure. The strong bonds in three dimensions make it hard ,high melting point solid. From this it is clear that CO2 is a gas and silicon dioxide is a solid . |