
Call 1800-123-2003

"Smartindia is the best online tool for school students." Ms Deepa Chandran, Principal Anchal
"Smartindia is one of the best supplementary education tool for school students." Ms Pooja, Principal
what do u mean by position isomers??? |
Position isomerismIt is due to the difference in the position of the substiuent atom or group or an unsaturated linkage in the same carbon chain.
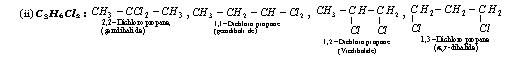 • Aldehydes, carboxylic acids (and their derivatives) and cyanides do not show position isomerism.
• Monosubstituted alicylic compounds and aromatic compounds do not show position isomerism.
• Structural isomers which differ in the position of the functional group are called regiomers. For example,
|
