1.a) The first law of thermodynamics for closed systems was originally
induced from empirically observed evidence, however, it is now taken to
be the definition of heat via the law of conservation of energy and the
definition of work in terms of changes in the external parameters of a
system.
For a given system we let ?E kin = large-scale mechanical energy, ?E pot = large-scale potential energy, and ?E tot = total energy. The first two quantities are specifiable in terms of appropriate mechanical variables, and by definition
-
-
-
-
-
-
-

For any finite process, whether reversible or irreversible,
-
-
-
-
-
-
-
-

- The first law in a form that involves the principle of conservation of energy more generally is
-
-
-
-
-
-
-
-

Here Q and W are heat and work added, with no restrictions as to whether the process is reversible, quasistatic, or irreversible.
1.b) Adiabatic process: no heat is transferred.
Isothermal process: occurs at a constant temperature.
It might appear that temperature cannot change if no heat is added, but
that is not the case. You can increase the temperature of a gas by
compressing it; even if the piston and cylinder are insulated against
heat transfer. Essentially, the compression process takes mechanical
work and converts it to both an increase in pressure and an increase in
temperature.
You can increase the temperature of a gas in an adiabatic process if you do some mechanical work on the gas.
2.c)
In thermodynamics, the work involved when a gas changes from state A to state B is simply
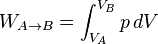
For an isothermal, reversible process, this integral equals the area under the relevant
pressure-volume isotherm, and is indicated in blue in the figure (at the
bottom right-hand of the page) for an ideal gas. Again, p = nRT / V applies and with T being constant (as this is an isothermal process), we have:
 - for many systems, if the temperature is held constant, the internal energy of the system also is constant, and so
 . From First law of thermodynamics . From First law of thermodynamics 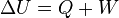 , so it follows that , so it follows that  for this same isothermal process. for this same isothermal process.
When no heat flows into or out of the gas because the temperature is
constant, then there is no work done. Thus, work=0 which means external
pressure is zero. This is called free expansion
|
